Brian Kennedy, CEO and Professor, Buck Institute for Research on Aging, discusses Drugs that Forestall Aging
-- Extending Healthspan.
Kennedy states that now, the pharmaceutical industry is doing a great job- keeping unhealthy people alive longer, though our goal is extending healthy lifespan, with a shorter period of decline. While outlining the seven determinants of aging, we note how interconnected they are.
Today though, we learn about interventions, both behavioral and small molecules. While dietary restriction in mice accounts for 30% longer lifespan, how can we translate that in humans?
Five classes of molecules (Rapamycin, Metformin, NSAIDS, Acarbose and STACs) that have been shown to increase healthy lifespan in mice.
Rapamycin is dose dependent and a 30% lifespan increase in females, 22% in males at the current highest recommended dose has been noted.
A clinical study of healthy people performed in New Zealand by Novartis, was a 6 week long trial looking at dosing of Rapamycin. One aspect that was reviewed was the response to influenza vaccination- an improved immune response with all doses was noted, as the inhibition of MTOR1 kinase, improves immune function in the elderly. Hmm, Rapamycin dosing a few weeks prior to grandma's flu shot?
Metformin is another small molecule, currently approved for type 2 Diabetes and non alcoholic fatty liver disease. Metformin works by suppressing glucose production in the liver, while enhancing peripheral glucose intake. A retrospective study comparing sulphonyurea and metformin, show patients with Diabetes on Metformin have a survival that is higher than patients without Diabetes and on Metformin (what?!)
Worried you are taking too many NSAIDS? Never fear, enhanced longevity has been noted in mice given NSAIDs, such as aspirin, ibuprofen, and COX-2 inhibitors. True, there are side effects, some of which may be avoided using a transdermal delivery method, which allows for the quick absorption of the drug while avoiding the GI system.
I look forward to learning more about these interventions, and which has the highest efficacy.
Friday, August 21, 2015
Thursday, August 20, 2015
Advances in Stem Cell Applications
Jeff Karp, Associate Professor, Brigham and Women’s Hospital, Harvard Medical School, presents Towards Accelerated Medical Innovation.
After receiving a letter from a pediatric cardiothoracic surgeon, who was seeking a way to repair septal defects in children, Karp was inspired to develop a better way to increase survival in this population. Due to constant beating of the heart, along with the flow of blood, this makes for a difficult surgical environment, one which sutures and staples are not a good fix. Hence, the development of a surgical glue and patch that would be able to withstand the wet condition caused by blood flow, along with withstand the constant movement required to move blood throughout the body were design considerations.
Using rats and pigs, the team was able to apply a patch and wet resistant glue with success. Considering the wide range medical implications of this glue, Karp founded Gecko Biomedical, a company whose "proprietary technology platforms are fully synthetic bioinspired light-activated tissue adhesives with strong adhesive and sealing capacity. The adhesives have unique chemical and physical properties, including high viscosity, hydrophobicity and on demand curing. These features allow them to be delivered through minimally invasive procedures to challenging wet environments, with no requirement for tissue drying prior adhesive application."
Steve Oh, Director, Agency for Science Technology and Research, wraps up the session, presenting on Stem Cell Bioprocesses and Senescence.
With an ultimate goal of growing enough cells in an affordable way to patch up one square-centimeter of damaged heart muscle following a heart attack. Rather than culturing cells on round, level Petri dishes, it was suggested perhaps they could attempt to develop them on minor polystyrene dabs known as micro-carriers.
Petri dishes typically fit less than 100,000 cells, a miniscule sum when stacked against the 2 billion muscle cells that make up the heart or the 100 billion red platelets expected to fill a sack of blood. This new methodology could possibly deliver cells in larger numbers, to make them more practical for therapy. This new process is an exponential improvement from previously used techniques.
 |
| Dr. Jeff Karp |
After receiving a letter from a pediatric cardiothoracic surgeon, who was seeking a way to repair septal defects in children, Karp was inspired to develop a better way to increase survival in this population. Due to constant beating of the heart, along with the flow of blood, this makes for a difficult surgical environment, one which sutures and staples are not a good fix. Hence, the development of a surgical glue and patch that would be able to withstand the wet condition caused by blood flow, along with withstand the constant movement required to move blood throughout the body were design considerations.
Using rats and pigs, the team was able to apply a patch and wet resistant glue with success. Considering the wide range medical implications of this glue, Karp founded Gecko Biomedical, a company whose "proprietary technology platforms are fully synthetic bioinspired light-activated tissue adhesives with strong adhesive and sealing capacity. The adhesives have unique chemical and physical properties, including high viscosity, hydrophobicity and on demand curing. These features allow them to be delivered through minimally invasive procedures to challenging wet environments, with no requirement for tissue drying prior adhesive application."
Next, we hear from Ivan Wall, Senior Lecturer & Undergraduate Admissions Tutor, Department of Biochemical Engineering, University College London, with a presentation titled Measuring and Enhancing MSC
Product Characteristics.
 |
| Dr. Ivan Wall |
With the study goal to measure cell qualities that are required for maintenance at the site of injury after therapeutic delivery. Cells were cultured under typical best practice. The effect was evaluated, and measures were performed in low oxygen (2%) as an in vitro model of physiologic oxygen pressure at damage locales. The impact of chemokine preconditioning with SDF1 was additionally evaluated. Findings showed that transient development brought about expanded cell connection however diminished rate of movement, while connection and relocation of patient-inferred bone marrow mononuclear cells was exceedingly heterogeneous.
Decreased oxygen impeded MSC connection, though not movement. The essential useful reactions of MSCs needed for maintenance and engraftment adjust quickly, even over a generally short extension period. This needs watchful thought when growing cells to accomplish clinical quantities for treatment.
 |
| Dr. Steve Oh |
Steve Oh, Director, Agency for Science Technology and Research, wraps up the session, presenting on Stem Cell Bioprocesses and Senescence.
With an ultimate goal of growing enough cells in an affordable way to patch up one square-centimeter of damaged heart muscle following a heart attack. Rather than culturing cells on round, level Petri dishes, it was suggested perhaps they could attempt to develop them on minor polystyrene dabs known as micro-carriers.
This procedure had been utilized in the past to mass-produce infection contaminated cells for the vaccine industry.
Petri dishes typically fit less than 100,000 cells, a miniscule sum when stacked against the 2 billion muscle cells that make up the heart or the 100 billion red platelets expected to fill a sack of blood. This new methodology could possibly deliver cells in larger numbers, to make them more practical for therapy. This new process is an exponential improvement from previously used techniques.
Therapeutic Approaches: Advances in Stem Cell Research
Kicking off the concurrent session for Advances in Stem Cell Research, Jeanne Loring, Professor, Scripps Research Institute presents Using Genomics to Improve Stem
Cell Therapy.
Human pluripotent stem cells have the astounding ability to grow undifferentiated and form into each cell type in the body. The mission of stem cell research is to propel human undifferentiated cells by the use of effective new cutting edge advances. Loring's lab takes a multifaceted approach to stem cell research, and today she discusses findings in her research, with a focus on the genomics of stem cells. Lessons learned regarding the safety of stem cell transplants from long term culture shows that to maintain stability, hPSC's should be expanded for banking using manual passaging and feeder layers. Additionally, lowest possible passage cells should be used, along with the use of quality assays to assure the right cell type with no deleterious mutations.
Next, Evan Snyder, Director, Center for Stem Cell and Regenerative Medicine, presents Using Stem Cells to Discover Therapeutic Pathways, Targets & Drugs. A pediatrician by background, one area of his research involves how neural stem cells self-renew and differentiate into neurons, astrocytes, and oligodendrocytes.
Human pluripotent stem cells have the astounding ability to grow undifferentiated and form into each cell type in the body. The mission of stem cell research is to propel human undifferentiated cells by the use of effective new cutting edge advances. Loring's lab takes a multifaceted approach to stem cell research, and today she discusses findings in her research, with a focus on the genomics of stem cells. Lessons learned regarding the safety of stem cell transplants from long term culture shows that to maintain stability, hPSC's should be expanded for banking using manual passaging and feeder layers. Additionally, lowest possible passage cells should be used, along with the use of quality assays to assure the right cell type with no deleterious mutations.
Next, Evan Snyder, Director, Center for Stem Cell and Regenerative Medicine, presents Using Stem Cells to Discover Therapeutic Pathways, Targets & Drugs. A pediatrician by background, one area of his research involves how neural stem cells self-renew and differentiate into neurons, astrocytes, and oligodendrocytes.
Searching for novel approaches to treating bipolar disorder. We know, for example, that lithium takes a shot at bipolar. Could we, as a result, pry into the can to uncover what is going on?
One of the objectives of lithium seems, by all accounts, to be CRMP2, a particle inside the neuron, that, in addition to other things, is essential to dendrite advancement. Dendrites are the sharp projections from neurons that get neurotransmitters from close-by axons stretching out from different neurons.
Just to make this absolutely befuddling, CRMP2 likewise underpins axonal development.
"Excitotoxicity" can meddle with the ordinary compound procedures of CRMP2. CRMP2 is getting phosphorylated excessively. This implies the biochemical "on-off" switch goes haywire, with unwanted downstream effects.
As indicated by Dr. Snyder, lithium seems to bring down the level of phosphorylation. Only time will tell if we will one day be able to make genetic modifications for those afflicted with these neuro disorders.
One of the objectives of lithium seems, by all accounts, to be CRMP2, a particle inside the neuron, that, in addition to other things, is essential to dendrite advancement. Dendrites are the sharp projections from neurons that get neurotransmitters from close-by axons stretching out from different neurons.
Just to make this absolutely befuddling, CRMP2 likewise underpins axonal development.
"Excitotoxicity" can meddle with the ordinary compound procedures of CRMP2. CRMP2 is getting phosphorylated excessively. This implies the biochemical "on-off" switch goes haywire, with unwanted downstream effects.
As indicated by Dr. Snyder, lithium seems to bring down the level of phosphorylation. Only time will tell if we will one day be able to make genetic modifications for those afflicted with these neuro disorders.
The Potential Impact of the 21st Century Cures Act on Healthcare Translation
As we wind down the day on Wednesday, a panel discussion with Anthony Atala (Wake Forest University), John Trojanowski (Professor of Geriatric Medicine and Gerontology, Perelman School of Medicine,
University of Pennsylvania) and David DiGiusto, (Executive
Director, Laboratory for Cell and Gene
Medicine, Stanford Health Care), moderated by David Brindley, Research Fellow, University of Oxford/Center for the Advancement of
Sustainable Medical Innovation.

Of course, we all know this act still has to become a law before Approximately $1.9B is to be allocated for funding complex research in medicine.
Many challenges remain, though, despite the potential influx of funds. The regulatory process is still long and costly, with an average of 15 years and $1B to bring to market. Additionally, novel treatments and devices never before seen, means the FDA has to re-evaluate their current approval processes. To add insult to injury, DiGiusto notes bias from funding organizations towards newer, younger researchers, without a proven track record, hence losing the funding opportunities they need to move forward to next steps.
Trojanowski points out that at least 50% of people age 80 and older will develop Alzheimer's Disease. Considering the fact that we are living longer than ever, the drain on the global economy this would have is huge. By developing treatments or prevention strategies for this and other disease of age, we would essentially help save our economy at the same time.
Of those who have walked the "Valley of Death" (the time between research and commercialization when many do not receive adequate funding to commercialize), it is clear that a new business model should be considered. For example, a type of annuity payment, where companies would get paid as long as the treatment/medication worked, no payment for failed treatments. Of course, public-private partnerships are another popular funding model. Clearly, collaboration and common sense amongst regulatory bodies and researchers will be crucial to the success of commercializing life saving treatments.

With the introduction of the 21st Century Cures Act, one goal is promoting the development and speeding approval of new drugs and devices, and to persuade the FDA to consider nontraditional study designs. The Act does numerous things, including making exploration joint efforts less demanding; elevating treatments like biomarkers to upgrade customized medication medicines, focused at people and not only extensively at illnesses; transforming and streamlining clinical trials and making it less difficult and extravagant for organizations to offer medications for sale to the public; making motivators for creating medications for unprecedented yet lethal sicknesses; making an Innovation Fund to urge youthful researchers to do way breaking research; and putting more cash into both the National Institutes of Health and the Food and Drug Administration to make these developments work.
Of course, we all know this act still has to become a law before Approximately $1.9B is to be allocated for funding complex research in medicine.
Many challenges remain, though, despite the potential influx of funds. The regulatory process is still long and costly, with an average of 15 years and $1B to bring to market. Additionally, novel treatments and devices never before seen, means the FDA has to re-evaluate their current approval processes. To add insult to injury, DiGiusto notes bias from funding organizations towards newer, younger researchers, without a proven track record, hence losing the funding opportunities they need to move forward to next steps.
Trojanowski points out that at least 50% of people age 80 and older will develop Alzheimer's Disease. Considering the fact that we are living longer than ever, the drain on the global economy this would have is huge. By developing treatments or prevention strategies for this and other disease of age, we would essentially help save our economy at the same time.
Of those who have walked the "Valley of Death" (the time between research and commercialization when many do not receive adequate funding to commercialize), it is clear that a new business model should be considered. For example, a type of annuity payment, where companies would get paid as long as the treatment/medication worked, no payment for failed treatments. Of course, public-private partnerships are another popular funding model. Clearly, collaboration and common sense amongst regulatory bodies and researchers will be crucial to the success of commercializing life saving treatments.
Wednesday, August 19, 2015
Emerging Technological Trends in Regenerative Medicine
After a lovely lunch here at the SENS conference, we continue on with the Emerging Technological Trends in Regenerative Medicine track, and learn about Genome Editing with Engineered
Nucleases, presented by Fyodor Urnov, Senior
Scientist, Sangamo BioSciences Inc.
New antiviral medications and combination therapies tend to emerge every few years, with over 30 currently on the market. I don't even want to venture a guess as to how much money big pharma has made as a result, nor do I care to outline all of the potential side effects. Although grateful for the extended lifespan of those afflicted with HIV, I have often wondered... what if we could modify our immune system to be resistant to HIV?
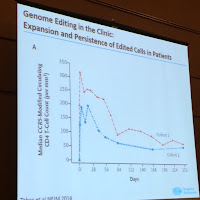
Guess what... we can. This technique has worked in six patients so far in phase II clinical trials. The technique involves harvesting a patient's T-cells, using genome-editing techniques to disrupt the gene that controls the receptor used by HIV to infect those cells, and returning the modified cells to the patient. By editing a major co-receptor of HIV, CCR5, in CD4 T cells, rendering the CCR5 permanently dysfunctional by a zinc-finger nuclease.
The team is using the same genome-editing technique to modify hematopoietic stem cells ex vivo, with the goal of treating hemoglobinopathies such as sickle-cell anemia and transfusion-dependent beta-thalassemia.
I look forward to following his teams work.
New antiviral medications and combination therapies tend to emerge every few years, with over 30 currently on the market. I don't even want to venture a guess as to how much money big pharma has made as a result, nor do I care to outline all of the potential side effects. Although grateful for the extended lifespan of those afflicted with HIV, I have often wondered... what if we could modify our immune system to be resistant to HIV?
Guess what... we can. This technique has worked in six patients so far in phase II clinical trials. The technique involves harvesting a patient's T-cells, using genome-editing techniques to disrupt the gene that controls the receptor used by HIV to infect those cells, and returning the modified cells to the patient. By editing a major co-receptor of HIV, CCR5, in CD4 T cells, rendering the CCR5 permanently dysfunctional by a zinc-finger nuclease.
The team is using the same genome-editing technique to modify hematopoietic stem cells ex vivo, with the goal of treating hemoglobinopathies such as sickle-cell anemia and transfusion-dependent beta-thalassemia.
 |
| Disease Targets for Genome Editing in Hematology |
SENS RB 2015: Therapeutic Approaches Track
I have been following the work of Dr. Anthony Atala since I was a nursing student in the late 90's. Today, he is presenting at the SENS Rejuvenation Biotechnology conference, with a talk titled Regenerative Medicine: Current Concepts and Changing Trends.
Dr. Atala serves as the Director at Wake Forest Institute of Regenerative Medicine, in addition to Chairing the Department of Urology at Wake Forest University.
Regarding his novel research and it successes, Dr. Atala discusses growing a urethra in a laboratory setting and its successful transplant into human subject. Utilizing collagen scaffolding (prior to 3D printing currently used) seeded with cells, the cells then vascularized and formed around the scaffold. Following this, his team then took it a step farther, growing and implanting a bladder using the same technique.
Dr. Atala serves as the Director at Wake Forest Institute of Regenerative Medicine, in addition to Chairing the Department of Urology at Wake Forest University.
 |
| Dr. Anthony Atala |
Regarding his novel research and it successes, Dr. Atala discusses growing a urethra in a laboratory setting and its successful transplant into human subject. Utilizing collagen scaffolding (prior to 3D printing currently used) seeded with cells, the cells then vascularized and formed around the scaffold. Following this, his team then took it a step farther, growing and implanting a bladder using the same technique.
 |
| Luke M., bladder transplant recipient |
Then things get really interesting... turns out the DOD has provided funding for development of another organ, one that soldiers may lose in war... the penis. I must say, after learning about the engineered vagina that was successfully developed and implanted, it only makes sense this would come next.
Seriously looking forward to what Wake Forest and Dr. Atala have in store for us next!
Subscribe to:
Comments (Atom)